COVID Vaccinations for Toddlers to Start After Juneteenth, White House Predicts
Ten million doses for kids under 5 became available Friday for states to order, said the White House. Shipment will begin following FDA authorization

Get stories like this delivered straight to your inbox. Sign up for The 74 Newsletter
Coronavirus vaccinations for children under 5 years old are likely to begin June 21, after the federal Juneteenth holiday, a top White House official said.
In a press conference Thursday, White House COVID Response Coordinator Ashish Jha outlined the possible timeline for when young children, the last group in the U.S. still ineligible for immunizations, could begin rolling up their sleeves.
Here are the key dates:
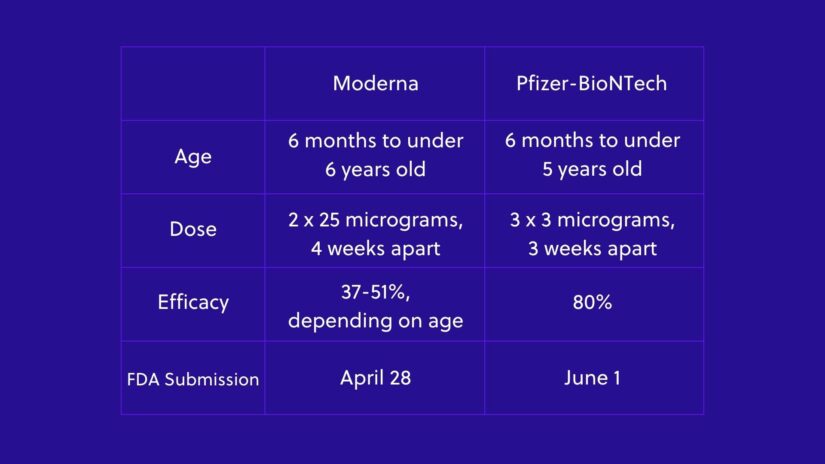
—June 1, Pfizer-BioNTech formally asked the Food and Drug Administration to grant emergency use authorization to their doses for kids under 5. Moderna submitted its application in late April for kids 6 months to 6 years old.
—June 3, states became able to order vaccine doses for kids under 5. A total of 10 million are currently available, the White House said.
—June 14 & 15, the FDA’s vaccine advisory committee is scheduled to meet to review the Moderna and Pfizer-BioNTech doses. Should the committee vote in favor of authorization, the White House expects the FDA to greenlight the vaccines in the days immediately after the meeting.
—June 18-20, if the FDA has authorized shots, doses will begin to arrive at doctors’ offices. The White House can begin sending vaccine shipments immediately following FDA authorization.
—June 21, after the long holiday weekend, if the previous steps proceed without setbacks, kids under 5 may begin receiving vaccine doses. The Centers for Disease Control and Prevention must also recommend the shots, but the agency typically follows the guidance of the FDA.
The White House has asked states to first provide vaccines to sites that can handle large volumes of supply, such as children’s hospitals. But as soon as Atlanta-based pediatrician Jennifer Shu receives the green light from local officials, she will order doses for her office.
“Parents have been asking me about vaccine availability for kids under 5 for several months. I plan to order them as soon as I get the notice from our health department,” she wrote in a message to The 74.
The White House also stressed that providers should offer vaccinations outside traditional working hours.
“We want to make this as easy as possible for working parents and their families,” said Jha.
The Department of Health and Human Services did not immediately respond to a question from The 74 asking how many doses have been ordered so far and by which states.
With COVID case counts once again high amid a second Omicron surge, the updated vaccine timeline for young kids appeared as a light at the end of the tunnel to many pandemic-weary parents.
“I teared up in the car today thinking about being able to get my kid vaccinated,” Marisol LeBrón, professor at UC Santa Cruz, wrote on Twitter.
Parents of young children awaiting vaccines for little ones have been on a months-long roller coaster that has repeatedly raised their hopes only to later send them crashing down. In late February, Pfizer-BioNTech first submitted a request asking the FDA to grant emergency authorization for a two-dose regimen of their vaccine for children 6 months to 4 years old, only to then withdraw the application just five days later.
Then in April, when Moderna was on the verge of submitting its EUA application for the age group, Politico reported that the FDA might postpone the review process until Pfizer’s shots were also ready, a reveal that angered many parents and spurred a congressional letter asking the agency to explain the reported delay. The FDA’s current timeline appears to confirm those speculations of a simultaneous review.
The Moderna and Pfizer-BioNTech doses have several differences. Moderna’s shots are a two-dose regimen spaced four weeks apart, while the Pfizer-BioNTech vaccine requires three doses each spaced three weeks apart. The Pfizer-BioNTech shots were 80% effective in clinical trials, while Moderna’s were 51% protective in toddlers 6 months to 2 years old and 37% protective in youngsters 3 to 5 years old.
Researchers believe both vaccines offer a strong defense against severe illness and hospitalization in the age group.
In a clip from the Thursday press conference that has circulated widely on Twitter, White House Press Sec. Karine Jean-Pierre cut off Jha before he could respond to a reporter’s question asking whether “all schools will and must be open this coming fall.”
Any speculations that the Biden administration would advise school closures next year, however, starkly contrast with the administration’s prior actions and messaging. Biden has continually underscored his commitment to keeping schools open and oversaw a push to 99% of schools offering in-person learning in his first months in office. Although early in the pandemic an in-person learning divide existed between red and blue states, virtually all school systems reopened their classrooms for the 2021-22 school year, regardless of their partisan leaning.
But with toddler vaccines possibly rolling out in just a few weeks, many older children have not yet been immunized. Just 29% of children 5 to 11 years old and 59% of youth 12 to 17 years old had received two vaccine doses as of June 1, according to data from the American Academy of Pediatrics. The rates that have remained nearly stagnant for months.
The winter’s massive Omicron surge demonstrated the importance of youth vaccination, said Shu, the Atlanta pediatrician. Children under 5 were hospitalized with the virus at five times the rate they were during the Delta surge, a study from the CDC recently found. And in February, the agency’s data revealed that 3 in 4 kids under 18 had been infected by the virus.
“The kids who are ending up in the hospital are more likely not to be vaccinated,” the doctor told The 74 in May.
Get stories like these delivered straight to your inbox. Sign up for The 74 Newsletter

;)
